Industry research highlights a harsh reality for pharmaceutical companies. Every day a clinical trial is delayed can cost close to $1 million in lost drug revenue, while trials themselves already cost around $50,000 per day to operate. Yet delays are becoming more common, not less. In 2024, over 20% of clinical trials reported delayed start dates, a steep rise compared to previous decades, driven largely by fragmented data systems and slow reporting processes rather than scientific setbacks.
For pharmaceutical manufacturers still relying on legacy tools like Crystal Reports, these delays compound quickly. Static, manually generated reports that take days to produce slow decision-making at critical moments, from safety monitoring to regulatory submissions. Migrating from Crystal Reports to Power BI is not just a visualization upgrade, but a strategic move to enable faster insights, real-time tracking, and compliant analytics across the clinical lifecycle.
In this blog, we explain how the Crystal Reports to Power BI migration enables faster, compliant insights across pharmaceutical operations.
Key Takeaways
1. Migrating from Crystal Reports to Power BI is a strategic shift for pharmaceutical companies to move from slow, static reporting to real-time, interactive insights that directly impact clinical timelines and patient safety.
2. Legacy reporting tools increase risk in pharma by delaying decision-making, creating data silos, and limiting visibility across trials, manufacturing, quality, and pharmacovigilance functions.
3. Power BI enables faster R&D, stronger compliance, and proactive safety monitoring through real-time dashboards, drill-down analysis, and audit-ready reporting aligned with GMP and 21 CFR Part 11 requirements.
4. A successful migration requires more than report conversion, including data modeling, security design, phased rollout, user training, and strong governance to ensure performance and adoption.
5. Real-world pharma use cases show measurable outcomes from Power BI adoption, including faster reporting cycles, improved operational efficiency, reduced BI costs, and better control over sensitive data.
6. Kanerika extends value beyond migration by building future-ready pharma data ecosystems with governed Power BI platforms, automated migration accelerators, and AI-driven analytics that support long-term innovation and regulatory confidence.

How Legacy BI Systems Are Slowing Down Pharma Development
Traditional reporting tools like Crystal Reports were built for a different era. With clinical trials spanning global sites, regulators demanding real-time monitoring, and AI reshaping drug discovery, these systems create more problems than they solve.
Here’s what’s breaking down:
- Static outputs: Crystal Reports deliver fixed snapshots, not dynamic dashboards. Clinical trial managers cannot filter, drill down, or compare trends in real time. These capabilities are essential for pharmacovigilance and adaptive trial management.
- Manual, error-prone processes: Legacy reporting requires hours of data extraction, spreadsheet manipulation, and manual verification. This drains resources and introduces compliance risks.
- Persistent data silos: These tools connect to individual databases, keeping R&D, manufacturing, quality, and commercial data isolated. Cross-functional visibility becomes nearly impossible.
- No AI integration: Legacy systems cannot feed real-time data to machine learning models or surface AI-driven predictions. Pharma companies miss out on predictive analytics that could accelerate development timelines and improve patient outcomes.
In an industry where delays cost millions and patient safety is paramount, outdated BI infrastructure is no longer just inefficient. It’s a competitive liability.
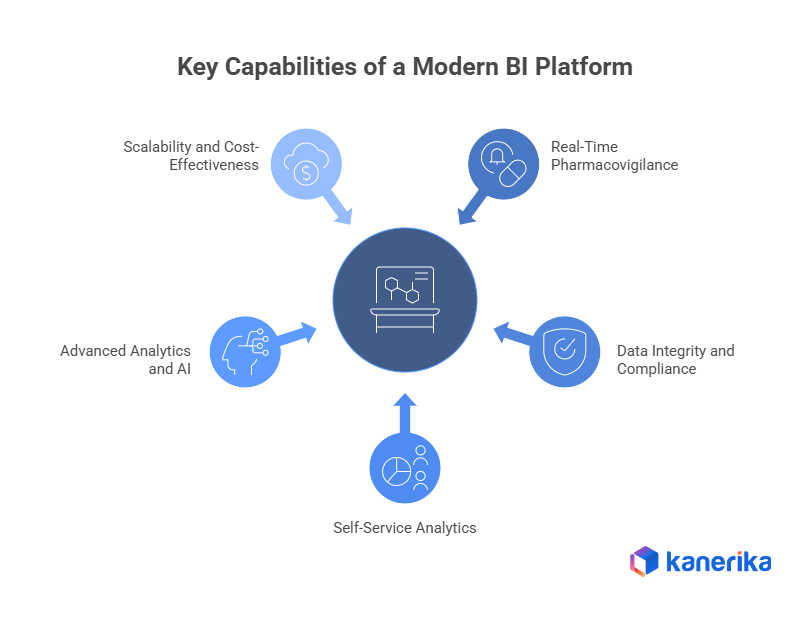
Pharma Data Modernization: Key Capabilities of a Modern BI Platform
Microsoft Power BI turns static data into dynamic, actionable information. It helps companies make better decisions about drug development and run their operations more smoothly. It is not just another reporting tool. Think of it as a tactical platform that lets you understand how your entire operation works and respond to business challenges before they become problems.
1. Real-Time Pharmacovigilance and Interactive Dashboards
Interactive Power BI dashboards show adverse event rates by drug, dose, patient demographics, or geography. They update automatically as new data comes in. Clinical trial managers use these dashboards to watch patient recruitment, check how sites are performing, track safety signals, and manage data quality. The nice thing is that teams can click on aggregate views and drill down to individual patient records. This makes investigations much faster and more targeted.
Pfizer Pharmaceuticals, one of the world’s largest pharmaceutical companies, began using Power BI for real-time trial monitoring. Their clinical operations teams got instant access to patient safety data across hundreds of trial sites worldwide. Instead of waiting for scheduled reports, teams could spot safety issues as they happened.
2. Better Data Integrity and Regulatory Compliance
Power BI helps companies stay compliant with key features that match what regulators require.
- Following GMP rules and ALCOA+ principles: Power BI connects with Manufacturing Execution Systems and ERP systems, giving real-time visibility into production processes. Dashboards track batch records, equipment calibration schedules, environmental monitoring, and how deviations are handled against GMP standards. Using structured data models and automatic refresh schedules makes it much easier to follow ALCOA+ principles.
- Automated reports for FDA, EMA, and 21 CFR Part 11: Power BI takes care of the routine compliance reports that regulatory bodies require. After initial setup and validation, data models and reports update on their own schedule, which cuts down on human error. When set up properly with the right supporting systems, Power BI can handle electronic signatures and full audit trails that meet 21 CFR Part 11 requirements.
- Getting audit-ready faster: With all critical data brought together and shown consistently in Power BI, preparing for audits takes much less time. Auditors can access secure, read-only interactive reports, dig into specific data points, verify compliance measures, and trace the source of data. This kind of transparency builds trust with regulators and shows strong control environments.
3. Self-Service Analytics That Empowers Teams
Power BI puts data in the hands of people across the organization. Scientists build custom analyses and explore data without waiting for IT to create tickets. They test hypotheses and get results faster. Sales teams track market trends, monitor territory performance, and review efficacy data directly. This lets them adjust strategies based on what is happening right now. When every department can work with data instead of just asking for reports, decisions happen faster, and everyone feels more confident about what they are doing.
A major pharmaceutical company in New Jersey used Power BI to change how its marketing and sales teams got market intelligence. Before, they waited weeks for the analytics department to create custom reports. With Power BI, sales leadership could instantly see product performance by territory, customer segment, or time period. This lets them respond to market changes much faster.
4. Advanced Analytics and AI Capabilities
Power BI connects directly with Microsoft Azure AI services, opening up sophisticated applications.
- Drug discovery: AI algorithms process complex genomic data to find potential candidates, predict how well compounds might work, and identify new biomarkers.
- Supply chain optimization: Predictive analytics forecast demand more accurately, optimize inventory across global networks, and spot potential disruptions before they happen.
- Patient outcomes analysis: Real-world evidence analysis helps understand how drugs work in broader populations, which can refine treatment protocols and point toward personalized medicine opportunities.
5. Scales Well and Costs Less in the Cloud
Power BI is built for the cloud, so it scales easily from small datasets to petabytes. Whether a company is managing data from one clinical trial or its entire global enterprise, Power BI handles it. The pay-as-you-go model means companies only pay for what they use, reducing upfront infrastructure costs. Pharma companies can grow their analytics capabilities without making big capital investments.
SoftwareOne worked with Adamed Pharma to build a fully automated forecasting solution using Azure and Power BI. The centralized data approach saved the company a lot of time and resources while making forecasts more accurate. Within a few months, they went from manual spreadsheet planning to automated, data-driven forecasting.
Power BI in Action: Real Results for Pharmaceutical Companies
1. Clinical Trial Management and Real-Time Patient Data Analysis
Large Clinical Research Organizations running multiple global trials use Power BI dashboards to track recruitment progress, monitor adverse events by study arm, compare site performance, and view key performance indicators, such as how quickly data queries are resolved. This real-time visibility helps spot bottlenecks, keeps patients safer, and speeds up trial completion.
When teams can see trial data in real time, they can fix issues before they become bigger problems. Site coordinators catch enrollment challenges early. Medical monitors review safety data on an ongoing basis. Project managers keep stakeholders informed without waiting for monthly status reports.
2. Supply Chain Visibility and Demand Forecasting
Power BI brings together data from ERP systems, logistics, warehouse management, and external market sources for end-to-end visibility. Dashboards track inventory levels, monitor cold chain integrity for biologics, forecast demand based on seasonal trends or public health data, and flag potential delays or stock-out risks.
Pharmaceutical companies that handle temperature-sensitive products depend heavily on cold chain monitoring. Power BI dashboards track shipments in real-time, alert teams to temperature problems, and keep historical data for compliance reporting. This level of visibility used to cost a lot of money with custom solutions.
3. Manufacturing Quality Control and Deviation Management
Power BI works with MES and LIMS systems to monitor critical process parameters, track batch yields, and manage deviations. Interactive dashboards help quality assurance teams spot out-of-spec result trends, find root causes faster, and handle corrective and preventive actions effectively. This keeps product quality high and maintains GMP compliance.
These dashboards help manufacturing teams notice trends that might indicate equipment problems or material quality issues. By catching trends early, companies can act before batch failures happen. This saves millions in potential waste and rework.
4. Drug Safety and Pharmacovigilance Reporting
Pharmacovigilance teams pull adverse event reports from multiple sources and identify trends in real time. Users filter interactively by drug, symptom, demographics, dosage, or time period, which helps detect signals and assess risks faster for regulatory reporting.
How quickly safety teams can spot and investigate potential signals matters a lot for patient safety. Power BI shortens the time between data entry and actionable insight. This helps companies meet their pharmacovigilance obligations while keeping patients safe.
5. R&D Pipeline Optimization
Power BI shows the entire drug development pipeline, tracking progress across phases, monitoring budget adherence, and identifying bottlenecks or resource constraints. Researchers analyze experimental data, compare how well compounds perform, and decide which projects to move forward with based on the data.
The Fortune 500 pharmaceutical company in New Jersey used Power BI to transform its strategic planning process. Leadership could see at a glance which programs were on track, which needed attention, and how to split resources across their research programs.
Crystal Reports to Power BI Migration 2025: Key Considerations
Learn how to migrate from Crystal Reports to Power BI for modern, interactive analytics.
How to Successfully Migrate from Crystal Reports to Power BI in Pharma
1. Start with Understanding What You Have
Before developing any code or dashboards, companies need to take stock of their existing Crystal Reports environment. This means that every report is catalogued, that there is an understanding of who is using the report and how often, and what reports are still relevant. Many organizations find they have accumulated hundreds of reports over the years, yet only a fraction are actually needed.
This is time-consuming inventory work that pays off in the long run. Teams can flex the migration efforts based on reports that are most critical to operations, most frequently used, or most problematic in the current state. Reports that are rarely used or contain duplicate information can be retired rather than migrated, saving effort and complexity.
2. Designing the Target State First
Jumping straight into conversion rarely goes well. Teams should take the time to design the Power BI environment. This includes making decisions on the overall architecture, how data will flow from source systems into the analytics layer, and how security will be structured.
Pharmaceutical companies need to consider regulatory requirements at this stage. Data retention policies, audit trail requirements, and access controls all need to be built into the solution from the beginning, not tacked on later. The target state should also define standards for dashboard design, naming conventions, and documentation maintenance.
3. Build Incremental Rather Than All at Once
Big-bang migrations in which everything is converted in a single release tend to be problematic. A phased approach is more effective. Teams can conduct a pilot with a small group of reports and users, collect feedback, make changes, and then scale up.
Each phase must provide a tangible value. Users should receive enhancements that are significant to their everyday work, rather than mere technical under-the-hood upgrades. This helps build confidence and produces internal advocates who help drive adoption throughout the organization.
SQL Server Data Migration Tools: Expert Guide for Enterprises
SQL Server data migration requires careful tool selection and strategic planning to avoid costly downtime and data loss
Critical Success Factors for Enterprise Data Migration
1. Executive Sponsorship and Appropriate Governance
Successful migrations usually have active executive sponsorship. Someone at the leadership level advocates the initiative, eliminates obstacles, and makes sure resources are available throughout the project. Without this, competing priorities have tended to put migration work on the back burner.
Clear governance is, of course, also important. Decisions on priorities, standards, and conflict resolution need to be made quickly. A governance structure that includes both technical and business stakeholders helps keep the project from being overly IT-focused or out of touch with operational realities.
2. Investment in Training and Change Management
Technical excellence is not paramount in driving adoption. Users need job-specific training rather than generic Power BI tutorials. A clinical researcher does not require the same training as a manufacturing analyst or a sales manager.
Change management is more than just training. It involves communicating why the migration is important, how it will impact different teams, and what support is available during the migration. Users who have a sense of the larger picture and who are supported are more likely to adopt new tools faster.
3. Partner Selection When Necessary
Many pharmaceutical companies have and work with external partners for at least part of their migration. The right partner brings experience with similar projects, knowledge of pharmaceutical regulations, and the ability to scale projects up or down based on needs.
A partner has to be chosen carefully. Companies should seek evidence of experience in the pharmaceutical or healthcare industries rather than general BI knowledge. References from similar clients, good communication regarding methodology, and realistic timelines are all important signals. The cheapest usually does not provide the best value when regulatory compliance and data accuracy are at risk.
4. A Realistic Timeline of Pharmaceutical Migrations
Migrating from Crystal Reports to Power BI in a pharmaceutical environment typically takes between 6 and 18 months, depending on the scope and complexity. Rushing through the process creates technical debt and compliance risks. Dragging it out indefinitely results in a loss of momentum and organizational support.
A reasonable timeline could be: two to three months for assessment and planning; three to six months for the build and validation of the core platform and initial reports; two to three months for pilot deployment and user feedback; and two to four months for phased rollout and optimization. These phases overlap to some extent, and the precise timing depends on organizational readiness and resource availability.
How to Migrate From Informatica to Databricks: Step-by-Step Migration Strategy
Explore the key benefits, common challenges, and a clear step-by-step migration process.
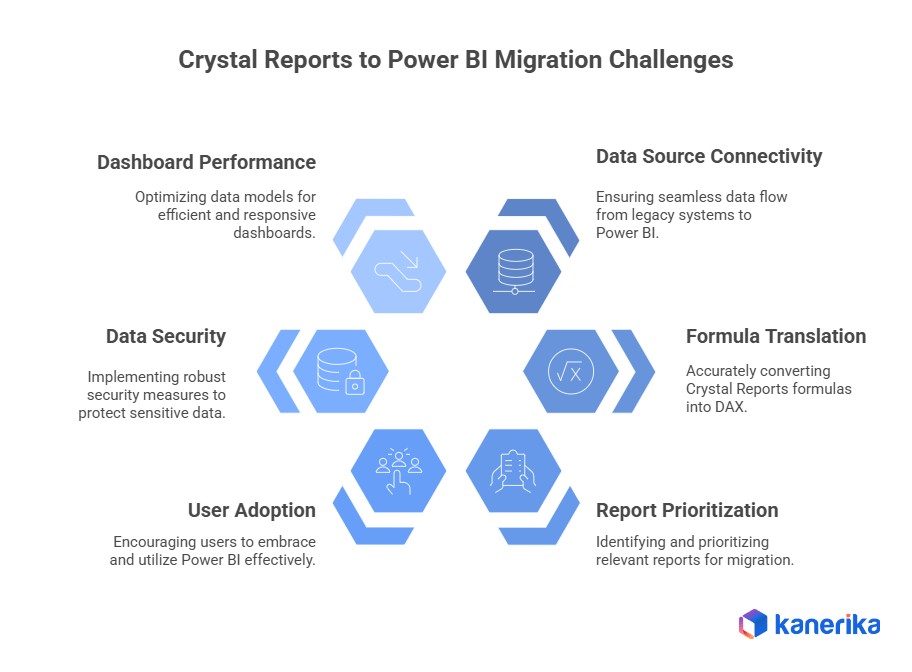
6 Key Challenges in Crystal Reports to Power BI Migration for Pharma
1. Connecting Data Sources Properly
Crystal Reports uses native database drivers to connect directly to source systems. Power BI works differently and requires more upfront setup. Many pharmaceutical companies have legacy systems with unusual databases or custom structures that lack straightforward Power BI connectors. Finding workarounds takes time. Sometimes companies need middleware or extra tools to bridge the gap. The risk is incomplete data connections leading to partial or inaccurate reporting.
2. Translating Formulas into DAX
This is one of the most time-consuming parts of any migration. Crystal Reports contains formulas built up over many years, often by different people. Some are deeply nested and complex. The person who wrote them may have left the company years ago. Power BI uses DAX and Power Query M, which work differently from Crystal’s formula language. Translating logic accurately requires someone who understands both systems. One small mistake can cascade into bigger problems across multiple reports.
3. Deciding What to Migrate First
Most companies have accumulated hundreds or thousands of reports over time. Not all are still relevant. Some duplicate information exists elsewhere. Others contain outdated calculations or connect to deprecated data sources. Identifying, cataloging, and prioritizing reports for migration is a major undertaking. Companies that try to migrate everything at once get overwhelmed. Companies that pick the wrong reports struggle to show quick value.
4. Getting Users to Actually Use Power BI
Technical success does not guarantee adoption. Many pharmaceutical professionals have used Crystal Reports for years and are comfortable with it, even if it is limited. Change feels uncomfortable, especially when people are already busy. Training helps, but it is not enough on its own. Users need to see how Power BI makes their specific job easier. Without proper change management, even well-executed migrations result in users reverting to old workarounds.
5. Keeping Data Secure and Meeting Pharma Requirements
Pharmaceutical companies handle highly sensitive information. Patient data, proprietary research, manufacturing processes, and financial information all need different levels of protection. Crystal Reports handles security at the report or user group levels, which is relatively simple. Power BI offers Row-Level Security and other advanced features, but configuring these correctly takes expertise. Getting it wrong exposes sensitive data or blocks users from information they need.
6. Making Sure Dashboards Perform Well
Crystal Reports generates static output. Power BI dashboards are interactive and refresh dynamically, which puts different demands on the underlying data model. Reports that worked fine in Crystal may load slowly or become unresponsive in Power BI if the data model is not optimized. Building efficient data models, using appropriate aggregation, and structuring relationships correctly requires experience. Companies that skip this step end up with dashboards that frustrate users with slow load times.
Real World Success: How Dr. Reddy’s Transformed with Power BI
The Challenge
Dr. Reddy’s handled data across Oracle, SQL Server, file systems, and Hive. Their systems held hundreds to thousands of tables, with data volumes up to 2 TB. Fragmented data slowed decision-making and made it difficult to track sensitive information such as PHI and PCI. The organization needed one reliable view of all operational and research data to improve speed, accuracy, and governance.
The Solution
Kanerika unified more than fifteen data sources into a single Power BI platform. The team added governance patterns to classify sensitive information, created custom security rules, and built real-time monitoring for operations and data quality. Power BI became the single source of truth, supporting analytics across R&D, manufacturing, quality, and business teams.
The Impact
Kanerika’s Power BI implementation delivered clear improvements supported by available case study data:
- 45% better operational performance with a unified analytics platform
- 30% lower BI costs through simplified architecture and governance
- 70% faster reporting cycles for real-time decision-making across teams
- Easier PHI and PCI tracking with improved sensitive data classification supporting CCPA and HIPAA
- Stronger AI readiness with high-speed pipelines for large compound datasets
Dr. Reddy’s moved from scattered, slow reporting to a unified data environment that supports faster decisions, stronger security, and scalable pharmaceutical innovation.
Beyond Migration: Building a Future-Ready Pharma Data Ecosystem with Kanerika
Migrating to Power BI is just the start. The real value comes from building data ecosystems that actually work for the long haul. These systems drive innovation, keep operations running smoothly, and stay compliant with regulations. Kanerika uses Microsoft Purview to set up data governance that covers mapping, cataloging, lineage tracking, and ownership. For pharma companies, this means tracing critical data from clinical trials through manufacturing and the supply chain. This level of transparency makes regulatory audits much smoother. Kanerika also configures Power BI to meet requirements for ISO 27001, SOC II, HIPAA, and GDPR, so everything stays compliant and ethical.
AI and machine learning are shaping the future of pharma. Power BI, paired with Kanerika’s AI expertise, opens up possibilities that were previously unattainable. Our Agentic AI predicts clinical trial dropout rates, flags early signs of manufacturing defects, or forecasts drug demand based on disease trends. Our Generative AI sums up lengthy clinical study reports and regulatory submissions into brief, readable documents. It also pulls key data from messy lab notes and research proposals, making R&D decision-making a lot faster.
Picking the right partner makes all the difference. Kanerika has spent decades working with pharmaceutical companies, so we understand 21 CFR Part 11, GMP, HIPAA, and GDPR inside and out. Our solutions actually work and pass audits. Our proprietary migration tools automate up to 80% of conversion tasks, cutting down manual work while keeping data intact. We also help set up Power BI Centers of Excellence and run training programs for everyone from R&D scientists to sales leaders, so adoption sticks and value keeps growing.
Migrate to Power BI for Smarter Analytics and Real-Time Insights!
Partner with Kanerika for Seamless Migration Services.
FAQs
What are the main benefits of migrating from Crystal Reports to Power BI for pharmaceutical companies?
Migrating to Power BI offers real-time interactive dashboards, enhanced regulatory compliance (21 CFR Part 11, GMP, HIPAA), robust self-service analytics, improved clinical trial data visualization, and seamless AI/ML integration. It replaces static manual reports with dynamic, actionable intelligence, accelerating drug development and ensuring patient safety.
How does Power BI ensure regulatory compliance in pharmaceutical settings?
Power BI for pharmaceutical analytics supports compliance through robust data governance features, secure data access (Row-Level Security), comprehensive audit trails, and Microsoft Purview integration. Kanerika’s KANComply solution automates compliance checks and documentation generation, helping pharmaceutical companies meet strict electronic records, data integrity, and patient privacy requirements.
What specific challenges does Kanerika address during Crystal Reports to Power BI migration?
Kanerika tackles converting intricate Crystal Reports formulas into Power BI DAX and Power Query M, re-engineering fragmented data models into optimized Star Schemas, implementing granular Row-Level Security for sensitive data, and efficiently managing large, undocumented report inventories. Our FLIP Migration Accelerators automate many tasks, ensuring accuracy and speed.
What is Kanerika's FLIP Migration Accelerator?
The FLIP Migration Accelerator is Kanerika’s proprietary toolset for Fast, Lean, Intelligent, and Precise migrations. It automates up to 80% of the Crystal Reports to Power BI conversion process, preserving complex business logic, reducing manual effort and errors, and ensuring zero data loss. This leads to faster project completion, lower costs, and guaranteed data integrity.
Can Power BI integrate with existing pharmaceutical systems?
Yes, Power BI integrates with Manufacturing Execution Systems (MES), Laboratory Information Management Systems (LIMS), Clinical Data Management Systems, and ERP platforms like SAP. Kanerika specializes in building robust, real-time data connections ensuring all critical operational, quality, and clinical data flows into Power BI for comprehensive analysis.
How does Power BI support advanced analytics and AI in drug discovery?
Power BI natively integrates with Microsoft Azure AI services, enabling pharmaceutical companies to leverage machine learning and custom generative AI solutions. This enables predictive forecasting for clinical trials, identification of new drug candidates through pattern analysis, supply chain demand prediction, and automated R&D document summarization.
What is the role of data governance in Kanerika's Power BI solutions?
Data governance is a cornerstone of Kanerika’s pharmaceutical Power BI solutions. We implement robust frameworks using Microsoft Purview and our specialized KANGovern, KANComply, and KANGuard tools. This ensures end-to-end data integrity, complete traceability, consistent data definitions, full regulatory compliance, and enhanced security against unauthorized access.
How long does a typical Crystal Reports to Power BI migration take?
Migration duration varies based on report quantity and complexity, data landscape size, and specific compliance requirements. However, Kanerika’s FLIP Migration Accelerators significantly reduce typical timelines, often automating tasks that traditionally take weeks or months into days or hours, leading to faster deployment and quicker value realization.
What training and support does Kanerika offer post-migration?
Kanerika provides comprehensive post-migration support including tailored, role-based training programs for all user levels—from basic Power BI navigation for business users to advanced DAX and data modeling for super-users and IT teams. We also assist in establishing Power BI Centers of Excellence for ongoing optimization and future analytical growth.
What are the main risks of delaying Power BI migration in pharmaceuticals?
Delaying migration prolongs reliance on outdated, static reporting, leading to increased compliance risks, significant operational inefficiencies from manual processes, and critical missed innovation opportunities in R&D, manufacturing, and patient care. The inability to access real-time, actionable insights severely hinders rapid decision-making, compromises patient safety, and erodes competitive advantage.